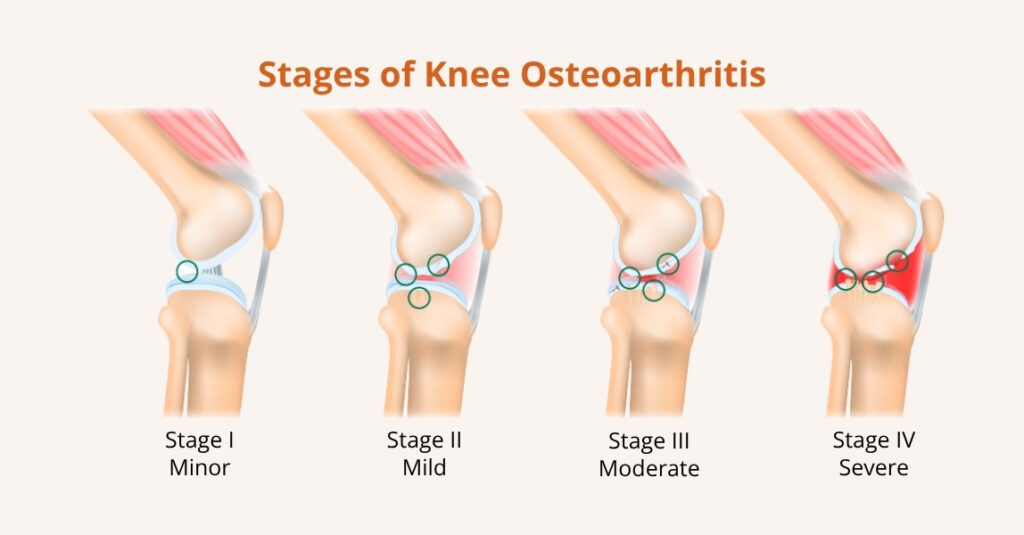
If your knee hurts, the thought of a full knee replacement can feel big, scary, expensive, and life-changing.
The good news is that a total knee replacement is not the only path.
Many people can ease pain, regain function, and stay active for years with other approaches that delay or even avoid major surgery.
Alternatives range from simple self-care and physical therapy to injections, braces, and newer minimally invasive or joint-preserving procedures.
These options can reduce pain, improve how you move, and, in some cases, protect the joint so you can keep doing the things you love.
However, which option is right depends on the extent of knee damage, your age, activity level, overall health, and your goals.
This article walks through both non-surgical and minimally invasive/joint-preserving options, clearly showing what each can do, its limitations, and the types of patients who typically benefit.
What are Knee Replacement Alternatives?
Knee replacement alternatives are treatments that help reduce knee pain and improve movement without needing a total knee replacement.
These options focus on managing symptoms, supporting the joint, and preserving as much of the natural knee as possible. They’re often used when someone wants to avoid major surgery, isn’t ready for it yet, or only has early-to-moderate joint damage.
Knee replacement alternatives are designed to:
- Reduce pain and inflammation: They help reduce swelling in the knee, ease stiffness, and make daily activities more comfortable.
- Improve mobility and function: By strengthening surrounding muscles or improving joint lubrication, these treatments help the knee move more smoothly.
- Delay or avoid knee replacement surgery: For many people, especially those with moderate arthritis, alternatives can buy valuable time before surgery is needed.
- Preserve natural knee structure: Instead of replacing the entire joint, these approaches aim to protect existing cartilage, bone, and ligaments for as long as possible.
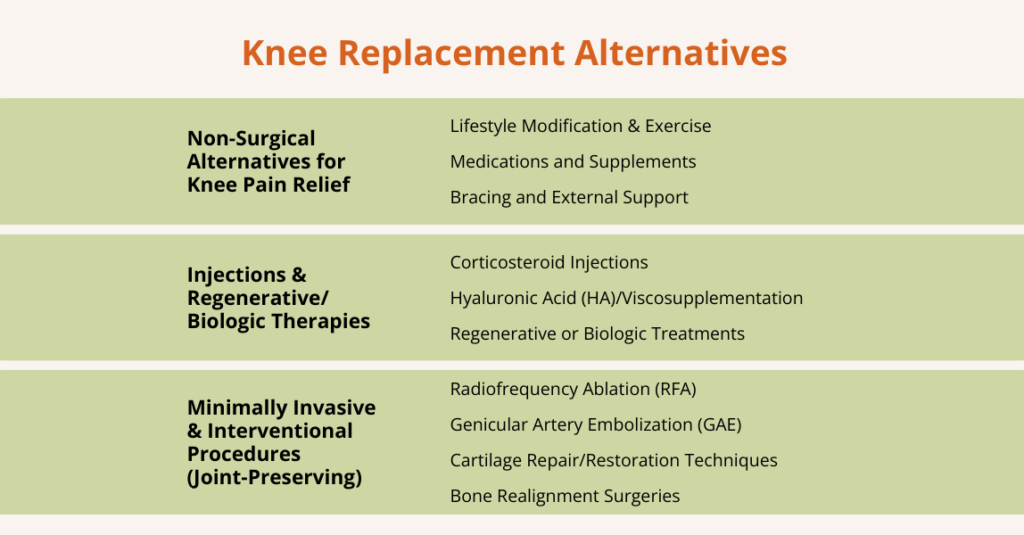
Non-Surgical Alternatives to Knee Replacement
Non-surgical treatments are often the first-line options for managing knee pain, especially in people with early or moderate knee problems. These approaches focus on reducing pain, improving mobility, and preserving joint health without surgery.
Lifestyle Modification & Exercise
One of the most effective ways to manage knee pain without surgery is through lifestyle changes and targeted exercises. These strategies focus on reducing stress on the joint, improving muscle support, and maintaining knee mobility.
- Weight Management: Losing excess weight reduces stress on your knee joints. Even a small amount of weight loss can significantly ease pain and slow further joint damage.
- Low-Impact Exercise: Activities such as walking, cycling, swimming, water-based exercise, or stationary biking help maintain mobility and keep joints moving without overloading them.
- Strengthening & Stability: Guided physical therapy and exercises targeting the quadriceps, hamstrings, and hip stabilizers strengthen muscles around the knee.
- Activity Modification: Avoiding high-impact activities such as running or jumping, and replacing them with knee-friendly routines, helps minimize wear and tear while still allowing you to stay active.
Medications and Supplements
For many people, medications and supplements can help manage knee pain alongside lifestyle changes and exercise.
Non‑Steroidal Anti-Inflammatory Drugs (NSAIDs), such as ibuprofen, are commonly used to relieve pain and reduce inflammation. They are effective for short-term symptom management and can help you stay more active.
A systematic review of 146 studies found that most (over 90%) reported positive outcomes for supplements such as glucosamine and Chondroitin sulfate in human joint pain and osteoarthritis, and that these supplements were generally well tolerated.
However, another study found that long-term NSAID use was associated with a significantly greater likelihood of worsened symptoms, including increased pain, stiffness, and disability, compared with non‑users.
That said, NSAIDs are not suitable for everyone and can carry risks, including gastrointestinal irritation or cardiovascular concerns, especially with long-term use. For this reason, medical supervision is important to ensure safe use.
Bracing and External Support
Knee braces are a simple way to support the joint, reduce pain and stiffness, and help you move more confidently. Many people with knee arthritis find them especially helpful for symptom relief and safer activity.
Here’s how it helps:
- Reduce pressure on parts of the knee joint
- Improve alignment and stability
- Decrease pain, stiffness, and improve function
Despite this, braces and external support are not a guarantee. Complications are uncommon but can include skin irritation, pressure sores, or nerve compression.
Injections & Regenerative/Biologic Therapies
Injections and biologic treatments aim to reduce knee pain, improve the joint environment, and help repair or slow the degeneration of tissues.
These therapies are often used when non‑surgical measures (like exercise, weight management, bracing) are not enough, or when someone wants to delay surgery while preserving the joint.
Types of injections & biologic options include:
Corticosteroid Injections
These are anti-inflammatory injections used to calm flare‑ups of arthritis or inflammation inside the knee joint. They can provide short‑ to moderate-term pain relief.
Hyaluronic Acid (HA)/Viscosupplementation
HA injections (joint lubrication shots) provide joint lubrication, reducing friction between joint surfaces. This can ease pain and improve mobility, especially in cases of osteoarthritis.
Regenerative or Biologic Treatments
Platelet-rich plasma (PRP) or stem-cell (mesenchymal stem cell, MSC) injections use the patient’s own biological material to support the knee joint.
These treatments aim to reduce inflammation, support tissue health, and in some cases encourage healing or slow cartilage degeneration.
While outcomes vary, these treatments focus on maintaining knee function and delaying the need for surgery.
Minimally Invasive & Interventional Procedures (Joint‑Preserving)

These treatments are designed for people who want pain relief and better knee function without going straight to major surgery like a total knee replacement.
Radiofrequency Ablation (RFA)
Radiofrequency Ablation (RFA) is a minimally invasive pain-relief procedure that targets the small sensory nerves around the knee, called genicular nerves.
These nerves carry pain signals from the knee to the brain. In RFA, a specialized needle delivers controlled heat via radiofrequency waves to “deactivate” these nerves, preventing them from sending strong pain signals.
No incisions are usually done under local anesthesia, and most people return to normal activity within a day or two. Many patients experience relief for 6–12 months, sometimes longer.
For this reason, it is useful for delaying or avoiding knee replacement, especially in people who are not ready or not good candidates for surgery.
Genicular Artery Embolization (GAE)
Genicular artery embolization (GAE) is a modern, minimally invasive procedure used to reduce knee pain caused by osteoarthritis. In osteoarthritis, the joint lining becomes inflamed and develops tiny blood vessels that contribute to pain.
During GAE, a doctor (usually an interventional radiologist) guides a small catheter into the blood vessels around the knee and releases tiny particles that block these extra vessels. By reducing abnormal blood flow, inflammation decreases, which can lead to less pain and improved mobility.
GAE is usually recommended for people who:
- Have moderate to severe knee osteoarthritis
- Still have significant pain even after trying treatments like medications, physical therapy, weight loss, or injections.
- Are not ready, not suitable, or not willing to undergo knee replacement surgery
Therefore, GAE is a promising option for people wanting relief without major surgery.
Cartilage Repair/Restoration Techniques
Cartilage repair procedures are designed to fix or regrow the smooth cartilage that covers the bones inside the knee.
When only a small area of cartilage is damaged, typically from an injury or early wear and tear, these techniques can help restore the surface and protect the joint.
Here’s how these techniques work
- One common method is Autologous Chondrocyte Implantation (ACI). In this procedure, a surgeon takes a small sample of healthy cartilage cells from your knee, grows them in a lab, and then implants them back into the damaged area.
- Other modern approaches use scaffolds (specialized materials placed inside the defect) or combine scaffolds with biologic therapies to promote new cartilage growth.
These treatments work best in younger, active patients or people who have localized cartilage defects, rather than widespread arthritis. Good knee alignment and healthy surrounding tissue are important for success.
Remember, cartilage repair is not suitable for everyone. Results can vary, recovery takes time, and these procedures are less effective when the entire joint is affected by arthritis.
Bone Realignment Surgeries
Bone realignment surgery, commonly called an osteotomy, is a joint-preserving procedure used when knee pain is caused by uneven weight-bearing. In many people, arthritis or wear-and-tear affects just one side of the knee.
This occurs when the leg is slightly angled inward (knock-knee) or outward (bow-legged), which increases pressure on one compartment of the joint.
An osteotomy reshapes or cuts the bone (usually the tibia or femur) to realign the leg. This shifts your body weight away from the damaged side and distributes it more evenly across the knee.
Different types of osteotomy include:
- High Tibial Osteotomy (HTO): Realigns the shin bone; commonly used when the inner (medial) side of the knee is worn down.
- Distal Femoral Osteotomy (DFO): Realigns the thigh bone; often used when the outer (lateral) side of the knee is affected.
- Opening-Wedge or Closing-Wedge Techniques: The surgeon either opens a small gap (and fills it with bone graft or plate) or removes a wedge of bone to achieve proper alignment.
Osteotomy is generally recommended for younger or middle-aged adults who still want to stay active but have knee pain from arthritis. It works best for people whose arthritis affects only one side of the knee rather than the entire joint.
Although osteotomy can be very effective, recovery typically takes several months because the bone needs time to heal after repositioning. Thus, it is not the best option for people with severe arthritis affecting the entire knee.
What Are the Pros and Cons of Knee Replacement Alternatives?
Understanding the pros and cons of knee replacement alternatives helps patients choose the option that best matches their condition, goals, and lifestyle.
| Pros | Cons/Limitations |
| Less invasive or non-invasive, lower risk than total knee replacement (less surgical trauma, lower infection risk). | Often, temporary relief may require repeat treatments (e.g., injections, RFA). |
| Shorter recovery time, faster return to routine activities than after major surgery. | Effectiveness varies by arthritis severity, alignment, weight, and overall joint condition. |
| Joint-preserving, keeps your natural knee anatomy and movement. | Regenerative therapies are still evolving; long-term evidence for cartilage regrowth is limited. |
| Flexible treatment combinations can combine therapies or use them step by step before surgery. | Not suitable for everyone, severe “bone-on-bone,” major deformity, or advanced arthritis may not respond well. |
| Can delay or avoid knee replacement; ideal for younger, active adults who want to protect their joint. | Access limitations. Advanced cartilage repair procedures may be available only at select centres. |
How to Decide if a Knee Replacement Alternative Is Right for You?
When facing knee problems or arthritis, it helps to follow a step-by-step, thoughtful decision-making process rather than jumping straight to major surgery.
The process often begins with the simplest, lowest-risk approaches and progresses only if symptoms persist or worsen, balancing benefit, risk, and each patient’s goals for their knee and lifestyle.
1. Start with Initial Evaluation & Conservative Management
At first, most patients begin with non-surgical care, such as lifestyle changes, physical therapy, or structured exercise, bracing or knee support, and, if appropriate, medications (such as NSAIDs).
These interventions aim to reduce joint load, strengthen surrounding muscles, improve mobility, and reduce pain, often with minimal risk and without surgery.
2. If Symptoms Persist or the Arthritis is Moderate
Consider additional therapies, such as injections or, if appropriate, regenerative/biologic therapies. These can sometimes provide greater relief or slow disease progression when conservative care alone isn’t sufficient.
3. If Pain/Function Limitations Despite Conservative & Injectables
In this case, more invasive procedures may be evaluated, such as nerve-targeting procedures or other pain-management approaches.
This makes sense, particularly if surgery is risky or if the patient wants to postpone a full knee replacement while still maintaining mobility and quality of life.
4. If Structural Damage Is Localized/Partial
In this situation, cartilage repair/restoration procedures, partial joint procedures, or bone-realignment surgery (osteotomy) may be considered, depending on alignment, cartilage health, and the patient’s activity goals.
5. Monitor & Reassess
Whatever path is chosen, conservative, injectable, interventional, or surgical, regular follow-up is essential. This includes clinical check-ups and imaging when necessary.
As arthritis progresses or the joint condition changes, treatment goals may shift, and at some point, a more definitive procedure (such as joint replacement) may become the best option.
How Do You Choose the Right Alternative to Knee Replacement?

Choosing a non-surgical, minimally invasive, or joint-preserving option depends on the person and the knee.
Here are some key aspects to consider when deciding which options fit best:
1. Stage of Disease
These alternatives work best when arthritis or cartilage loss is mild to moderate, meaning enough of the joint surface remains intact.
If the damage is limited, treatments like injections, bracing, cartilage repair, or osteotomy can relieve symptoms and protect the joint. When arthritis is widespread and severe, however, joint replacement is often the more reliable solution.
2. Age and Activity Level
Younger or more active patients often benefit most from joint-preserving and regenerative options, as preserving natural cartilage and bone helps them remain active longer.
Older patients or those seeking a definitive, long-lasting fix may lean toward replacement, but age alone shouldn’t rule anyone out; overall health and goals matter, too.
3. Patient’s Health and Surgical Risk
For people with other health problems (for example, heart or lung disease), or those who are poor candidates for major surgery, minimally invasive choices are attractive because they carry lower surgical risk and shorter recovery.
These options give symptom relief while avoiding the stress of a major operation.
4. Symptom Severity and Goals
If the main goal is to reduce pain, improve function, and delay a major operation rather than immediately replace the knee, conservative and interventional options are appropriate.
Patients with severe, constant pain that limits daily life despite other treatments may still need replacement sooner.
5. Patient Preferences
Patient values and priorities matter. Some people prefer less invasive treatments first, even if results might be temporary, to avoid major surgery.
Others prefer a single, durable solution and accept the tradeoffs of joint replacement. However, good decision-making balances the likely benefits and risks and considers how each option fits the patient’s lifestyle and goals.
Frequently Asked Questions (FAQs)
Is there an alternative to knee replacement surgery?
Yes, there are several alternatives to knee replacement, depending on the severity of your knee arthritis and your goals. The main types of alternatives include:
Conservative / Non-Surgical Treatments
- Weight management
- Physical therapy and targeted exercise
- Knee braces and supports
- Medications like NSAIDs
- Activity modification
Injection Therapies
- Corticosteroid injections
- Hyaluronic acid (viscosupplementation)
- PRP or stem-cell (MSC) injections
Interventional/Minimally Invasive Procedures
- Radiofrequency Ablation (RFA)
Joint-Preserving Surgical Options
- Cartilage repair/restoration procedures
- Osteotomy (bone realignment surgery)
What is the best alternative to knee replacement?
There isn’t a single “best” alternative to knee replacement; the right choice depends on your knee’s condition, age, activity level, and personal goals. For early or moderate arthritis, starting with conservative measures like weight management, physical therapy, bracing, and NSAIDs is usually effective. If pain persists, injections such as corticosteroids, hyaluronic acid, or biologic treatments like PRP can provide additional relief. For patients who want pain control without major surgery, minimally invasive options like radiofrequency ablation or genicular artery embolization may help. Younger or active patients with localized cartilage damage or malalignment may benefit from joint-preserving procedures such as cartilage repair or osteotomy.
How can I fix my knees without surgery?
You can manage knee problems without surgery by managing your weight, engaging in low-impact exercise, and undergoing physical therapy to strengthen and support the joint. Knee braces can improve alignment and reduce pain, while medications or injections (NSAIDs, corticosteroids, hyaluronic acid, or PRP) help control inflammation.
For persistent pain, minimally invasive procedures like radiofrequency ablation or genicular artery embolization may be options. In some cases, joint-preserving surgeries such as cartilage repair or osteotomy can preserve function and delay replacement. Regular monitoring and activity adjustments are key to staying active and managing symptoms.
What is the new procedure instead of knee surgery?
The newest non-surgical procedure for knee pain is Genicular Artery Embolization (GAE). In this minimally invasive treatment, a doctor blocks the small blood vessels (genicular arteries) that supply the inflamed tissue. By reducing abnormal blood flow, GAE helps decrease inflammation, relieve pain, and improve function, especially for people with moderate knee arthritis who want to avoid or delay knee replacement. The procedure is performed through a small incision, usually in an outpatient setting, and allows for a faster recovery than traditional surgery while preserving the knee’s natural structure.
Is there a way to avoid a knee replacement?
Yes, in many cases, you can avoid or delay a knee replacement. It depends largely on the severity of joint damage, your age, lifestyle, and how much you’re willing to invest in care and maintenance. Here’s how:
Lifestyle & Exercise: Lose excess weight, do low-impact exercises, and strengthen knee-supporting muscles.
Physical Therapy & Bracing: Stabilize the joint, improve mobility, and reduce pain.
Injections / Lubrication: Corticosteroids or hyaluronic acid to ease pain and improve joint movement.
Regenerative Therapies: PRP or stem-cell injections to reduce inflammation and support tissue healing.
Minimally Invasive Procedures: Options like Genicular Artery Embolization (GAE) or nerve-targeting treatments for pain management.
These approaches can help manage symptoms, preserve joint function, and delay surgery, depending on your knee’s condition and overall health.
How to avoid knee surgery naturally?
Many people can manage knee pain and protect their joints without surgery by making smart lifestyle choices and adopting natural strategies that reduce stress on the knee, strengthen supporting muscles, and improve joint health.
Here are some ways to avoid knee surgery naturally:
- Keep a Healthy Weight: Less weight reduces pressure on the knees and slows joint wear.
- Strengthen Supporting Muscles: Strong quadriceps, hamstrings, and hip muscles help stabilize the knee.
- Low‑Impact Exercise: Walking, cycling, swimming, or yoga maintains mobility without overloading the joint.
- Improve Flexibility: Gentle stretching helps maintain knee flexibility and reduce stiffness.
- Use Heat & Ice Therapy: Ice reduces swelling; heat relaxes muscles and improves blood flow.
- Anti‑Inflammatory Diet: Foods like fruits, vegetables, fish, nuts, and turmeric help reduce inflammation.
- Herbal Supplements: Turmeric, ginger, or omega‑3s may help decrease inflammation.
- Avoid High‑Impact Activities: Limit running, jumping, or deep squats that strain the joint.
- Wear Proper Footwear: Supportive shoes reduce joint stress and maintain alignment.
These approaches won’t reverse severe arthritis, but they can slow progression, ease pain, and help you stay active longer.
Conclusion
If you’re looking to manage knee pain without jumping straight to surgery, there are many options available.
From lifestyle changes and exercises to injections, biologic treatments, and minimally invasive procedures, these approaches can help reduce pain, improve movement, and protect your natural knee.
These alternatives are especially helpful if your arthritis is mild to moderate, if you’re younger or active, or if you just want to delay or avoid major surgery.
Remember, there’s no single best solution; the right choice depends on your knee, your health, and your goals.
The best way to decide is to discuss with your healthcare team. Your doctor, physiotherapist, or interventional specialist can help you determine which options are right for you and in the right order so that you can stay active and comfortable for as long as possible.